1.
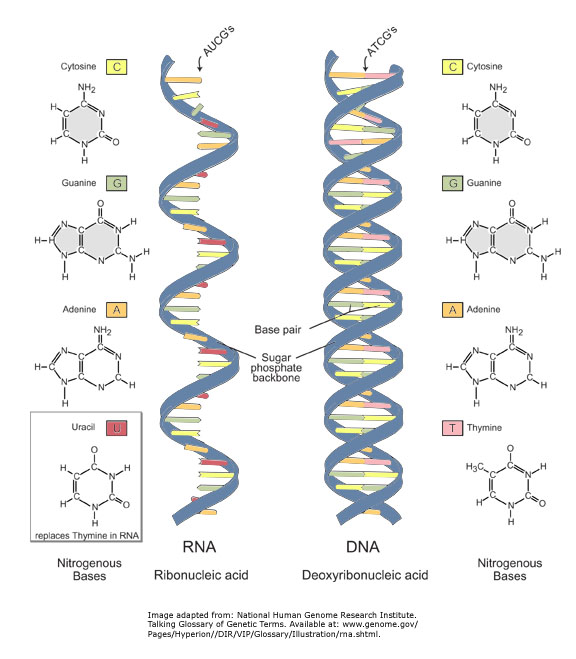
RNA
Ribonucleic acid (RNA) is one of the three major macromolecules (along with DNA and proteins) that are essential for all known forms of life.
Like DNA, RNA is made up of a long chain of components called nucleotides. Each nucleotide consists of a nucleobase (sometimes called a nitrogenous base), a ribose sugar, and a phosphate group. The sequence of nucleotides allows RNA to encode genetic information. For example, some viruses use RNA instead of DNA as their genetic material, and all organisms use messenger RNA (mRNA) to carry the genetic information that directs the synthesis of proteins.
Like proteins, some RNA molecules play an active role in cells by catalyzing biological reactions, controlling gene expression, or sensing and communicating responses to cellular signals. One of these active processes is protein synthesis, a universal function whereby mRNA molecules direct the assembly of proteins on ribosomes. This process uses transfer RNA (tRNA) molecules to deliver amino acids to the ribosome, where ribosomal RNA (rRNA) links amino acids together to form proteins.
The chemical structure of RNA is very similar to that of DNA, with two differences--(a) RNA contains the sugar ribose while DNA contains the slightly different sugar deoxyribose (a type of ribose that lacks one oxygen atom), and (b) RNA has the nucleobase uracil while DNA contains thymine (uracil and thymine have similar base-pairing properties).
Unlike DNA, most RNA molecules are single-stranded. Single-stranded RNA molecules adopt very complex three-dimensional structures, since they are not restricted to the repetitive double-helical form of double-stranded DNA. RNA is made within living cells by RNA polymerases, enzymes that act to copy a DNA or RNA template into a new RNA strand through processes known as transcription or RNA replication, respectively.
2.
Cytosol

The cytosol or intracellular fluid (or cytoplasmic matrix) is the liquid found inside cells. It is the liquid of a cell, that is parted from other parts of the cell by cell walls, such as the mitochondrial matrix inside the mitochondrion. The entire contents of a eukaryotic cell within cell membrane, minus the contents of the cell nucleus, are referred to as the cytoplasm. In prokaryotes, most of the chemical reactions of metabolism take place in the cytosol, while a few take place in membranes or in the periplasmic space. In eukaryotes, while many metabolic pathways still occur in the cytosol, others are contained within organelles.
The cytosol is a complex mixture of substances dissolved in water. Although water forms the large majority of the cytosol, its structure and properties within cells is not well understood. The concentrations of ions such as sodium and potassium are different in the cytosol than in the extracellular fluid; these differences in ion levels are important in processes such as osmoregulation and cell signaling. The cytosol also contains large amounts of macromolecules, which can alter how molecules behave, through macromolecular crowding.
Although once thought to be a simple solution of molecules, multiple levels of organization exist in the cytosol. These include concentration gradients of small molecules such as calcium, large complexes of enzymes that act together to carry out metabolic pathways, and protein complexes such as proteasomes and carboxysomes that enclose and separate parts of the cytosol.
3.
Histone
In biology, histones are highly alkaline proteins found in eukaryotic cell nuclei, which package and order the DNA into structural units called nucleosomes. They are the chief protein components of chromatin, acting as spools around which DNA winds, and play a role in gene regulation. Without histones, the unwound DNA in chromosomes would be very long (a length to width ratio of more than 10 million to one in human DNA). For example, each human cell has about 1.8 meters of DNA, but wound on the histones it has about 90 millimeters of chromatin, which, when duplicated and condensed during mitosis, result in about 120 micrometers of chromosomes.
4.
London dispersion forces
London dispersion forces (LDF, also known as dispersion forces, London forces, induced dipole–induced dipole forces) is a type of force acting between atoms and molecules.[1] They are part of the van der Waals forces. The LDF is named after the German-American physicist Fritz London.
The LDF is a weak intermolecular force arising from quantum induced instantaneous polarization multipoles in molecules. They can therefore act between molecules without permanent multipole moments.
London forces are exhibited by nonpolar molecules because of the correlated movements of the electrons in interacting molecules. Because the electrons from different molecules start "feeling" and avoiding each other, Electron density in a molecule becomes redistributed in proximity to another molecule, (see quantum mechanical theory of dispersion forces). This is frequently described as formation of "instantaneous dipoles" that attract each other. London forces are present between all chemical groups and usually represent the main part of the total interaction force in condensed matter, even though they are generally weaker than ionic bonds and hydrogen bonds.
This is the only attractive intermolecular force present between neutral atoms (e.g., a noble gas). Without London forces, there would be no attractive force between noble gas atoms, and they wouldn't exist in liquid form.
London forces become stronger as the atom or molecule in question becomes larger. This is due to the increased polarizability of molecules with larger, more dispersed electron clouds. This trend is exemplified by the halogens (from smallest to largest: F2, Cl2, Br2, I2). Fluorine and chlorine are gases at room temperature, bromine is a liquid, and iodine is a solid. The London forces also become stronger with larger amounts of surface contact. Greater surface area means closer interaction between different molecules.
5.
van der Waals force
In physical chemistry, the van der Waals force (or van der Waals interaction), named after Dutch scientist Johannes Diderik van der Waals, is the sum of the attractive or repulsive forces between molecules (or between parts of the same molecule) other than those due to covalent bonds or to the electrostatic interaction of ions with one another or with neutral molecules.[1] The term includes:
* force between two permanent dipoles (Keesom force)
* force between a permanent dipole and a corresponding induced dipole (Debye force)
* force between two instantaneously induced dipoles (London dispersion force)
It is also sometimes used loosely as a synonym for the totality of intermolecular forces. Van der Waals forces are relatively weak compared to normal chemical bonds, but play a fundamental role in fields as diverse as supramolecular chemistry, structural biology, polymer science, nanotechnology, surface science, and condensed matter physics. Van der Waals forces define the chemical character of many organic compounds. They also define the solubility of organic substances in polar and non-polar media. In low molecular weight alcohols, the properties of the polar hydroxyl group dominate the weak intermolecular forces of van der Waals. In higher molecular weight alcohols, the properties of the nonpolar hydrocarbon chain(s) dominate and define the solubility. Van der Waals-London forces grow with the length of the nonpolar part of the substance.
6.

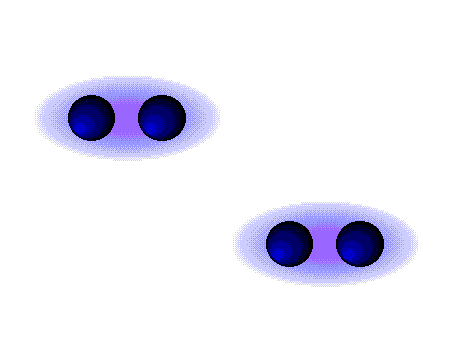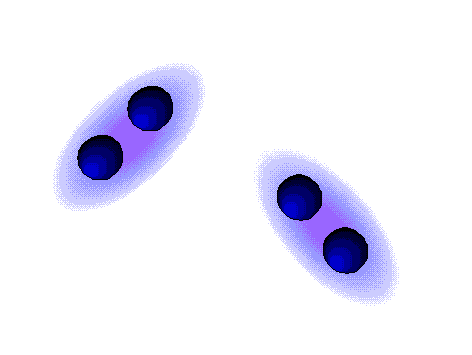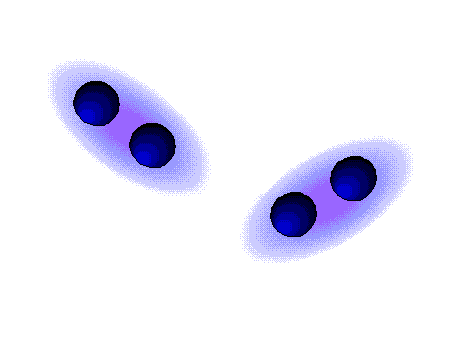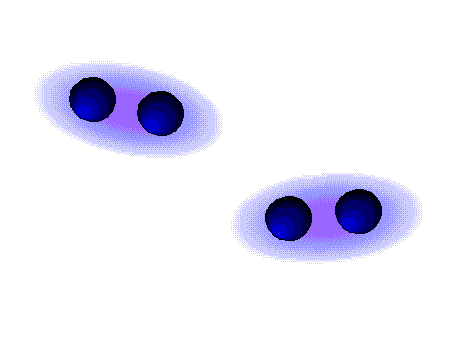
Dipole-Dipole Forces
Dipole-dipole forces are attractive forces between the positive end of one polar molecule and the negative end of another polar molecule. Dipole-dipole forces have strengths that range from 5 kJ to 20 kJ per mole. They are much weaker than ionic or covalent bonds and have a significant effect only when the molecules involved are close together (touching or almost touching).
The figures show two arrangements of polar iodine monochloride (ICl) molecules that give rise to dipole-dipole attractions.
Dipole-dipole attractions in ICl
Note:
* Polar molecules have a partial negative end and a partial positive end.
* The partially positive end of a polar molecule is attracted to the partially negative end of another.
* In a ICl molecule the more electronegative chlorine atom bears the partial negative charge; the less electronegative iodine atom bears the partial positive charge.
* The partially positive iodine end of one ICl molecule is attracted to the partially negative chlorine end of another ICl molecule.
A dashed line is used to represent an intermolecular attraction between molecules because these forces are NOT as strong as chemical bonds.
7.

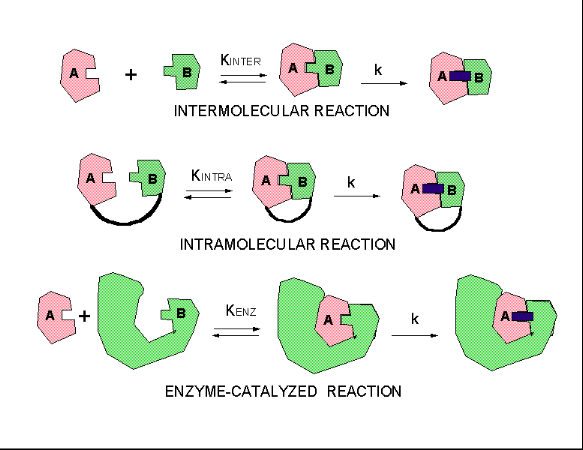
Intermolecular versus Intramolecular bonds
Intermolecular attractions are attractions between one molecule and a neighbouring molecule. The forces of attraction which hold an individual molecule together (for example, the covalent bonds) are known as intramolecular attractions. These two words are so confusingly similar that it is safer to abandon one of them and never use it. The term "intramolecular" won't be used again on this site.
All molecules experience intermolecular attractions, although in some cases those attractions are very weak. Even in a gas like hydrogen, H2, if you slow the molecules down by cooling the gas, the attractions are large enough for the molecules to stick together eventually to form a liquid and then a solid.
In hydrogen's case the attractions are so weak that the molecules have to be cooled to 21 K (-252°C) before the attractions are enough to condense the hydrogen as a liquid. Helium's intermolecular attractions are even weaker - the molecules won't stick together to form a liquid until the temperature drops to 4 K (-269°C)
8.
Cleavage Furrow
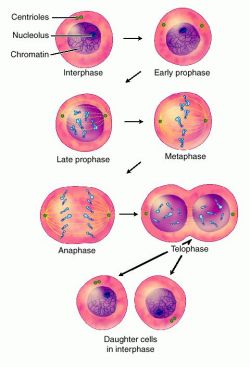
In cell biology, the cleavage furrow is the indentation that begins the process of cleavage, by which animal and some algal cells undergo cytokinesis. The same proteins responsible for muscle contraction, actin and myosin begin the process of forming the cleavage furrow. This can only happen in animal cells. Plant cells do not form a cleavage furrow. Instead, plant cells begin cytokinesis with the formation of a cell plate. The cleavage furrow begins on the outside of the cell and moves inward towards the center while the cell plate begins in the center and grows outward to meet the cell wall. Furrow appears because a ring of actin filament forms just inside the plasma membrane, in a plane that bisects the cell. Myosin binds to these actin filaments. Myosin moves the ring of actin filaments on the side of the plasma membrane, and the ring shrinks in size and tightens. The shrinking ring pulls the membrane with it because it is attached to the plasma membrane. The actin and myosin filaments continue to slide past each other, tightening the ring further, until the original membrane is pinched in two and cell division is complete.
9.
Genome
In modern molecular biology and genetics, the genome is the entirety of an organism's hereditary information. It is encoded either in DNA or, for many types of virus, in RNA. The genome includes both the genes and the non-coding sequences of the DNA/RNA.
Some organisms have multiple copies of chromosomes, diploid, triploid, tetraploid and so on. In classical genetics, in a sexually reproducing organism (typically eukarya) the gamete has half of the number of chromosome of the somatic cell and the genome is a full set of chromosomes in a gamete. In haploid organisms, including cells of bacteria, archaea, and in organelles including mitochondria and chloroplasts, or viruses, that similarly contain genes, the single or set of circular and/or linear chains of DNA (or RNA for some viruses), likewise constitute the genome. The term genome can be applied specifically to mean that stored on a complete set of nuclear DNA (i.e., the "nuclear genome") but can also be applied to that stored within organelles that contain their own DNA, as with the "mitochondrial genome" or the "chloroplast genome". Additionally, the genome can comprise nonchromosomal genetic elements such as viruses, plasmids, and transposable elements.[3]
When people say that the genome of a sexually reproducing species has been "sequenced", typically they are referring to a determination of the sequences of one set of autosomes and one of each type of sex chromosome, which together represent both of the possible sexes. Even in species that exist in only one sex, what is described as "a genome sequence" may be a composite read from the chromosomes of various individuals. In general use, the phrase "genetic makeup" is sometimes used conversationally to mean the genome of a particular individual or organism. The study of the global properties of genomes of related organisms is usually referred to as genomics, which distinguishes it from genetics which generally studies the properties of single genes or groups of genes.
Both the number of base pairs and the number of genes vary widely from one species to another, and there is only a rough correlation between the two (an observation known as the C-value paradox). At present, the highest known number of genes is around 60,000, for the protozoan causing trichomoniasis (see List of sequenced eukaryotic genomes), almost three times as many as in the human genome.
An analogy to the human genome stored on DNA is that of instructions stored in a book:
* The book (genome) would contain 23 chapters (chromosomes);
* each chapter contains 48 to 250 million letters (A,C,G,T) without spaces;
* Hence, the book contains over 3.2 billion letters total;
* The book fits into a cell nucleus the size of a pinpoint;
* At least one copy of the book (all 23 chapters) is contained in every cell of our body.
10.
germ cells
Germ Cells/Somatic Cells
In biology, germ cells are the cells that give rise to the gametes of organisms that reproduce sexually. In many animals, the germ cells originate near the gut and migrate to the developing gonads. There, they undergo cell division of two types, mitosis and meiosis, followed by cellular differentiation into mature gametes, either eggs or sperm. Unlike animals, plants do not have a germ line set aside in early development. Instead, germ cells can come from somatic cells in the adult floral meristem.
Somatic cells (diploid) are any cells forming the body of an organism, as opposed to germline cells. In mammals, germline cells (also known as "gametes") are the spermatozoa and ova which fuse during fertilization to produce a cell called a zygote, from which the entire mammalian embryo develops. Every other cell type in the mammalian body—apart from the sperm and ova, the cells from which they are made (gametocytes) and undifferentiated stem cells—is a somatic cell: internal organs, skin, bones, blood, and connective tissue are all made up of somatic cells.
The word "somatic" is derived from the Greek word sōma, meaning "body".












No comments:
Post a Comment